Inspire V Broadly Available in the United States
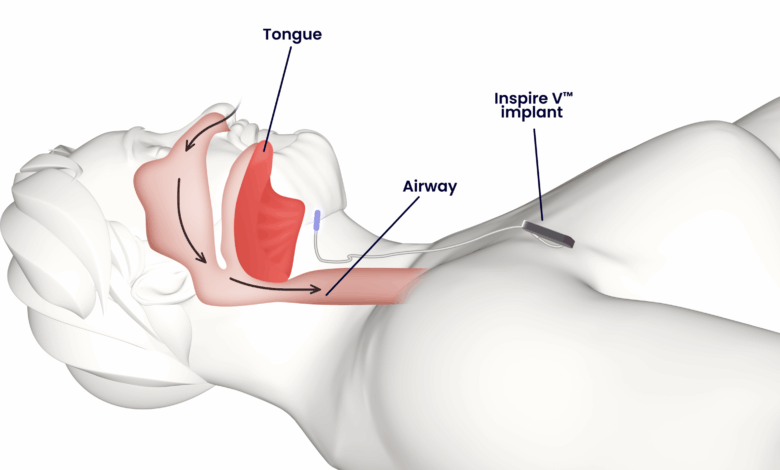
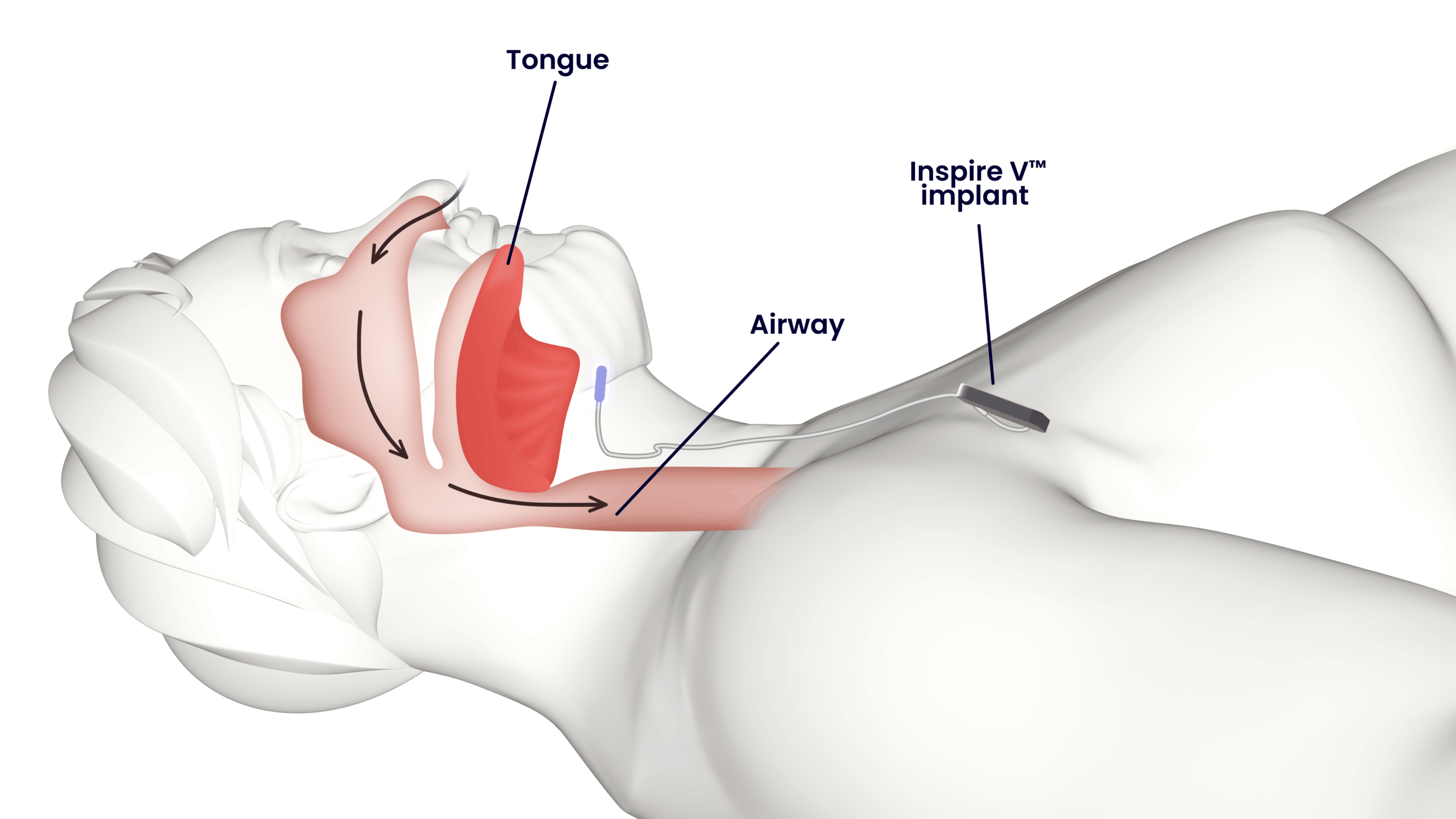
Inspire V, Inspire Medical’s newest technology for obstructive sleep apnea (OSA), is now broadly available in the United States. The Food and Drug Administration cleared the next-generation neurostimulator implant last year, and the device was in soft launch mode until recently as the company built sufficient inventory.
Inspire V streamlines the implant procedure by eliminating the need for a separate sensing lead. With a fully integrated respiratory sensor, Inspire V enables a shorter, simplified implantation process while offering enhanced patient comfort and more efficient long-term management.

“We are excited about the full launch of Inspire V across the United States,” says Inspire chairman and CEO Tim Herbert, via email. “Our new technology simplifies the procedure for providers by eliminating the sensing lead by incorporating sensing inside the neurostimulator, and further, offers features to enhance patient comfort. Inspire V also improves upon the already high reliability of our previous generations, and the team at Inspire continues to focus on patient outcomes through innovation. The Inspire V system is another positive step into the future.”
Otolaryngologist Paul T. Hoff, MD, MS, says via email, “Inspire V is the newest generation, and it brings me a lot of excitement to offer it to patients who qualify.” Hoff, who joined Inspire as vice president, senior medical director on April 21, adds: “As a surgeon, the elimination of the sensing lead, with a breathing sensor built into the implantable pulse generator, is a huge plus— simplifying the procedure, while added features to enhance comfort continues to build on Inspire’s mission to put patients first.”
The Inspire V system eliminates the need for physicians to place a separate sensing lead and instead allows for respiratory sensing to occur inside of the implant. This shortens and simplifies the procedure, making it easier for physicians while also enhancing patient comfort and allowing for more efficient patient management.
This year, Inspire also celebrates its 11th anniversary as the first FDA-approved implant to treat OSA. More than 100,000 patients worldwide have received Inspire therapy.



